First of all, since electrons are bound to an atom, we have energy quantization. They're not bound by any kind of actual barrier, but kept near the nucleus by the electric attraction between the nucleus and the electrons. Kept there in the same way that the nine planets stay near the sun instead of roaming the galaxy. The quantization is a bit more complicated than the example given on the last page, because an atom is three-dimensional, but the result is the same. The wave associated with the electrons in an atom can only take on certain shapes, and each shape has a specific amount of energy associated with it. To look at this in more detail, we'll consider the hydrogen atom. It's the simplest atom because it has only one electron, which quorbits a nucleus of one proton (and usually no neutrons).

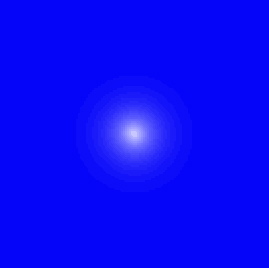
Now what you see here is sort of a cross section. That is, you have to imagine the picture rotated around the vertical axis. So the region inhabited by this electron looks sort of like a disk, but it should actually be sort of a sphere. This graph is for an electron in its lowest possible energy state, called its "ground state."
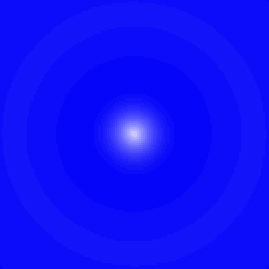 The electron "shown" here is in a higher energy state, or an "excited" state. Again, the electron is most likely to be near the nucleus, but notice the light ring near the outside, and the darker gap in between. Again, you have to rotate this around the vertical axis, so that that outer "ring" is really a spherical shell. If you think back to the wave diagrams for the particle in a box, remember that the higher energy states got progressively wavier. It's the same thing here. Also notice that in this higher energy state, the electron is likely to be farther away from the nucleus than in the ground state. Remember that potential energy is higher when the electron is farther from the nucleus.
The electron "shown" here is in a higher energy state, or an "excited" state. Again, the electron is most likely to be near the nucleus, but notice the light ring near the outside, and the darker gap in between. Again, you have to rotate this around the vertical axis, so that that outer "ring" is really a spherical shell. If you think back to the wave diagrams for the particle in a box, remember that the higher energy states got progressively wavier. It's the same thing here. Also notice that in this higher energy state, the electron is likely to be farther away from the nucleus than in the ground state. Remember that potential energy is higher when the electron is farther from the nucleus.
|
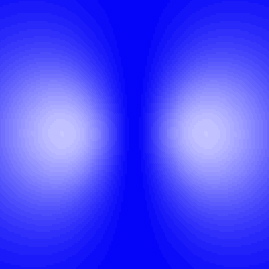 Here's another excited state, but this one's a little different. Notice that at the center, where the nucleus is, the picture is dark, indicating that the electron is unlikely to be there. The two light regions, where the electron is most likely to be found, are really just one region. Remember, you have to mentally rotate this around a vertical axis, so that in three dimensions the light region is really doughnut shaped.
Here's another excited state, but this one's a little different. Notice that at the center, where the nucleus is, the picture is dark, indicating that the electron is unlikely to be there. The two light regions, where the electron is most likely to be found, are really just one region. Remember, you have to mentally rotate this around a vertical axis, so that in three dimensions the light region is really doughnut shaped.
|
Each electron has a wave function, which describes where that electron is most likely to be found. The wave function depends on the energy of the electron. The higher the energy, the farther out from the center the electron is likely to be. Because all the electrons are confined to the area around the nucleus, energy is still quantized. That is, the energy of each electron can only take on certain values. However, when you put more than one electron in an atom, there's a new phenomenon that shows up.
When you lift up an object, and then drop it, it falls down. As it does this, its potential energy decreases. This is a general feature of all forces. Objects tend to lose potential energy whenever possible. When you apply this to an atom, you might guess that an electron might do the same thing. That is, if an electron is in a state with a high potential energy (such as the last two pictures above), it might drop down to a state with lower potential energy (such as the ground state, shown in the first picture). In fact, this happens. The electron in a hydrogen atom that's in an excited state will drop down to a lower energy state, and eventually to the ground state. This drop is called a "quantum jump." It results in a tiny flash of light (although usually it's a flash of light in a color that we can't see).
If an atom has lots of electrons, as most atoms do, you might then expect that all the electrons will drop down to the ground state and stay there. It turns out, though, that this doesn't happen. The reason it doesn't happen is that no two electrons can exist in the same quantum state. This is known as the Pauli exclusion principle. If a given state is occupied by an electron, all other electrons are excluded from that state. This means that an electron can't jump down to the ground state, if there's already an electron there. The result is that, while some of the electrons in an atom are in low energy states, others are forced by the exclusion principle to occupy higher energy states, farther out from the nucleus.
This turns out to be hugely important. You see, some of the most important properties of atoms have to do with how they behave with other atoms. That is, how they attract or repel each other. In other words, chemistry. And chemistry all has to do with how the outer, high energy electrons in an atom behave. The innermost, low energy electrons mostly just quorbit the nucleus, and don't have much to do with other atoms. It's the outer electrons of an atom that interact with the outer electrons of other atoms. And this determines how atoms hold together or fall apart.