Quantum physics is a rich and interesting field of physics, and I encourage you to learn all you can about it, just for fun. As an introduction, you might try "The Page of Uncertainty," by yours truly. In the meantime, here's a brief introduction to the most relevant features (for our purposes) of quantum physics.
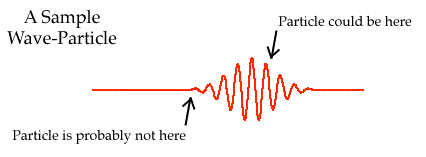
One of the central concepts in quantum physics is "wave-particle duality." As briefly as possible, here's what it means. Any particle, such as an electron or proton, has a wave associated with it. This wave will typically extend over only a small region, and the particle will be somewhere in that region. Exactly where in that region the particle is, is unknown. In fact, one can even go so far as to say that when the wave is spread out over a small region, the particle's location is undetermined. That is, the particle doesn't even have a definite location. This is part of what's meant by the famous "Heisenberg uncertainty principle."
However, we can always perform some sort of experiment to measure the location of the particle. The result of such a measurement will depend on the wave. That is, when we experimentally determine the particle's location, it's most likely to turn up where the wave has the largest oscillations. The size, or "amplitude" of the wave at any spot determines the probability of finding the particle there, should we decide to find it at all.

Until we measure the particle's location, it doesn't have a location. I realize this last sentence sounds a bit odd. (It sounds kind of zen, if, like me, you don't know anything about zen.)
So, you see, the electrons in an atom don't orbit the nucleus in elliptical paths. They don't follow any paths at all. They're in the neighborhood of the nucleus, but unless we actually perform some sort of experiment to find where they are, they aren't anywhere in particular. That's why I've got the red circle and slash through the picture of an atom on the opening page of this website. It's not like that picture at all. Unfortunately, most physics books still use the word "orbit" to describe what an electron does inside an atom. This isn't very accurate, but the problem is that there really isn't a word that describes what an electron is actually doing in an atom.
Well, this isn't quite true. You see, I've recognized this problem for some time now, and have addressed it by inventing my own word. The word is "quorbit," and I've been trying to get it into circulation for some time now. Here's what it means:
quorbit (kwor' bit) vt To be in the vicinity of, while lacking a definite location. As in, "The electrons in an atom quorbit the nucleus."If you would like to help me bring this fabulous new word into common usage, see the "quorbit" homepage.
But I digress. In order to understand what an electron does in an atom, that is, in order to understand an electron's quorbital motion, we need to look at a basic feature of quantum physics, called "quantization." The simplest example of quantization is a hypothetical example which physicists call the "particle in a box."