
(It's a law, so I figured I'd better make it look like one.)
So what does this mean? Really, it's just a summary of all that I've said so far. The entropy of a macrostate depends on the number of microstates that make it up. And since all microstates are equally likely, a macrostate with more microstates (higher entropy) is more likely than one with fewer microstates (lower entropy). The second law says that if a closed system is in a state with low entropy, it will naturally find a state with high entropy, if there's one available. (By "available", we mean an aerosol air freshener will spread out over the entire room only if you push down the button. If you don't push down the button, it can't get out of the can.)
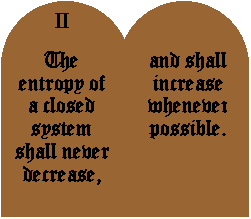
I should point out what we mean by a closed system. A closed system is a system to and from which no energy (especially heat) or matter (atoms and molecules, etc.) can flow. It's a system which has no interaction with anything outside it. This is sort of an idealization, since nothing in the universe can be completely shielded from its surroundings. On the other hand, the universe itself is probably a closed system (cosmologists do debate this, though) and so with that in mind, you can probably restate the second law, saying that the entropy of the universe never decreases, and increases whenever possible.
So now that we've condensed the whole discussion down to one sentence and
carved it into stone, we can look at the implications more deeply. For example,
the second law provides the answer to the question we posed earlier: Why do
things happen the way they do, and not in reverse? The answer is that as things
happen, the entropy of the universe increases. If they happened in reverse,
the entropy would decrease.
Let's have some examples. Entropy increases when...
... heat flows from a hot object to a cold object. (See "Entropy and energy" )And these are just a few examples, because the entropy of the universe increases in every process. (At least it never decreases.) And when the reverse of any of these processes occurs, entropy decreases.
... a gas flows from a container under high pressure, to a space at lower pressure. (For example, when you spray something out of an aerosol can, or when you let air out of a tire.) (See "Entropy and a box of gas" )
... heat is added to a substance. (Heat is energy. The more energy in a substance, the more ways you can distribute that energy. More ways = higher entropy. Think of it this way. Suppose you're passing out one dollar bills to a group of 10 people. If you only have $15, you're rather limited in the number of ways to distribute it. If you have $1,000,000 for 10 people, the permutations (microstates) are nearly endless! Now instead of dollars, think energy.)
... ice melts. (In ice, the water molecules are all locked into specific locations, while in water, they are free to wander throughout the liquid. This freedom means more microstates for water than ice.)
... water evaporates. (When water on a countertop evaporates, the water molecules are now free to wander throughout the entire room. Just as with our box of gas, the more space the molecules can wander through, the higher the entropy.)
But wait! I've put water in my freezer, and it comes
out ice!
The Page of EntRoPy
![]() Back to Dave's Physics Shack?
Back to Dave's Physics Shack?