Now the atom was already known to contain tiny particles called "electrons," which carry a negative electric charge. (We'll get to electric charge in the next page.) Rutherford found that the region at the center, which we now call the "nucleus," carries a positive charge. The electrons aren't in the nucleus, but instead surround it. So the atom is found to be in two parts: a tiny nucleus at the center, which contains a positive charge, and the surrounding region, which is sparsely populated by negatively charged electrons.
The nucleus is populated by two kinds of particles: positively charged "protons," and electrically neutral particles, called "neutrons." These particles are nearly identical to each other, except in electric charge. Protons and neutrons ("nucleons," as they're referred to collectively) are much heavier than electrons, and that's why most of the mass of an atom is in the nucleus.
Atoms are classified according to the number of protons in their nuclei. For example, any atom with six protons in its nucleus is a carbon atom. Any atom with one proton is a hydrogen atom. 26 protons means iron, 92 protons for uranium, and so on.
The number of neutrons in an atom can vary, but is typically close to the number of protons, for light elements (atoms with small numbers of protons). For example, the most common variety of carbon has six neutrons, in addition to its six protons. 6 protons + 6 neutrons = 12 nucleons, and so this variety is called Carbon-12. Many carbon atoms, however, have 8 neutrons. 6 + 8 = 14, so this type of carbon is Carbon-14, something you may have heard of in the context of radioactive dating, for example. Different varieties of the same element with different numbers of neutrons are called different "isotopes." As an example of a heavier element, lead atoms have 82 protons, and the most common isotope of lead has 126 neutrons. Heavy elements typically have many more neutrons than protons.
The number of electrons in an atom is typically the same as the number of protons, which makes the atom as a whole electrically neutral. (In a carbon atom, for example, the positive charge of six protons is exactly cancelled by the negative charge of six electrons.) Atoms with more electrons than protons, or vice versa, are electrically charged, and are called "ions."
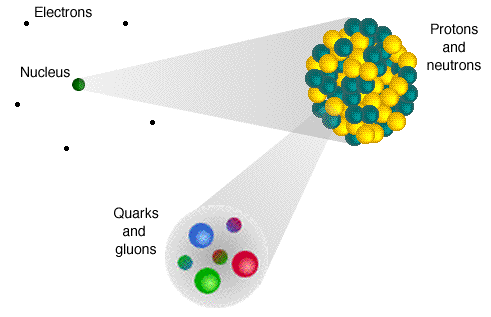
So we're left with the following picture, which should not be taken too seriously. It's only here to show you the levels of structure in an atom. It looks like an atom about as much as a weather map full of jagged lines, giant L's and H's, and a puffy cloud with a golden thunderbolt looks like the sky.

Anyway, quarks and gluons aren't made up of anything smaller that we can tell. Which means, that's it. Atoms are made up of electrons and nuclei. Nuclei are made up of protons and neutrons. Protons and neutrons are made up of quarks and gluons.
Where it stops, nobody knows.
* If you've seen the classic WKRP in Cincinnati episode in which Venus Flytrap explains the atom, you already have the gist of much of this page, and can continue to the next one if you like. Or, go back to the top of this one.