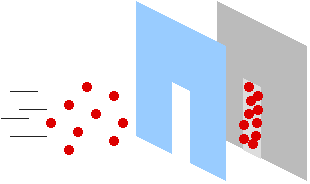
Suppose you have a whole bunch of ping-pong balls. You stand back about fifteen feet from a doorway, and one by one you dip the balls in paint and throw them through the door, at a wall about 5 feet past the door. Well, you'll get a bunch of colored dots on the wall, scattered throughout an area the same shape as the door you're throwing them through. This is how particles (such as ping-pong balls) behave.

On the other hand, waves don't behave this way. Think of water waves. When a wave encounters an obstacle, it goes around it and closes in behind it. When a wave passes through an opening, it spreads out when it reaches the other side. And under the right conditions, a wave passing through an opening can form interesting patterns on the other side, which can be deduced mathematically.
So here's what Young did. He took light, and shone it through a very narrow slit, and then shone that light through two very narrow slits, very close together. He then observed the result of this on a screen. Now if light is made up of particles, then the particles should pass straight through the slits and produce two light stripes on the screen, approximately the same size as the slits. (Just like the ping-pong balls in the picture above.) On the other hand, if light is a wave, then the two waves emerging from the two slits will interfere with each other and produce a pattern of many stripes, not just two. (Trust me on this, or I'll be forced to go through the math...)
![]()
The result? Young found the interference pattern with many stripes, indicating that light is a wave.
Later in the nineteenth century, James Clerk Maxwell (see Dave's Relativity Page) determined that light is an electromagnetic wave: a wave of oscillating electric and magnetic fields. When Heinrich Hertz experimentally confirmed Maxwell's result, the struggle to understand light was finished. Case closed.
The problem was that physicists had already applied the best of all their knowledge about thermodynamics and electromagnetism to this problem, and they didn't like the result. The result was that if you examined the spectrum from infrared light, through the visible spectrum (red through violet), and on into ultraviolet light, the intensity of the emitted light just goes up and up and up. This means that an object at any temperature (even if not completely black) radiates an infinite amount of energy, a result that clearly makes no sense, and was termed the "ultraviolet catastrophe."
In 1900 Planck discovered a way around this problem. He found that if light can only be emitted in small bursts, rather than a continuous wave, the resulting calculation not only made sense, but matched what people measured experimentally.
Albert Einstein carried Planck's discovery one step further when he applied it to another nagging inconsistency in physics, this one concerning the "photoelectric effect." When light is shone on a metal surface, electrons can be ejected from that surface. This is called the photoelectric effect. Without going into detail, if one assumes that light is a wave, as Young showed, then there are certain features of the photoelectric effect that simply seem impossible. What Einstein showed is that if one assumes that light is made up of particles (now called "photons"), and if these particles have the properties described by Planck for his small bursts of light, then the photoelectric effect all makes sense. When Robert Millikan performed experiments which confirmed some of Einstein's ideas, Einstein won the Nobel Prize (as did Millikan a couple years later).