But wait!
Okay. Here's where we're at. We've said that the entropy of the universe never decreases. (I've made amazingly convincing arguments for this by now!) We've said as an example, that the entropy of water increases when it melts, and decreases when it freezes. But we know that ice happens!
The key here is the word "universe". Entropy can decrease in some things, provided it increases in others. This is how ice can freeze. You put water at, say 40 degrees (Fahrenheit) into your 20 degree freezer. Heat flows from the water (which is warmer) to the rest of the freezer. When heat is added to something, its entropy increases. So the entropy of your freezer increases, while the entropy of the water decreases. As for the total amount of entropy, it increases. (Entropy increases when heat flows from a warm substance to a cold substance.)
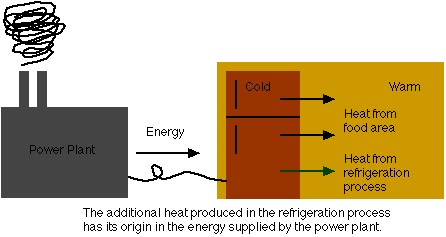
But it gets more interesting. Let's consider the refrigerator that's making your ice. The basic function of a refrigerator is to take heat out of its interior, and transfer it out the back into your kitchen (or wherever your refrigerator is). Notice which way the heat flows here. From the refrigerator (cold) to your kitchen (warm). Entropy increases when heat flows from warm to cold. So here, when heat flows from cold to warm, it must decrease! Another apparent violation of the second law of thermodynamics.
Well if this process happened by itself, the second law of thermodynamics would be violated. But it doesn't. Your refrigerator doesn't work unless you plug it in. When you plug it in, energy is delivered from some power plant to your refrigerator. This energy is used to drive a motor inside your fridge, and pump some fluid around or something. Whatever it is that refrigerators do when you plug them in. (Hey, I'm a theorist, okay?) But the byproduct of this is that more heat is produced and pumped into your kitchen. And more heat means an increase in entropy.
So if we only consider the heat pumped into your kitchen from the food storage area of your refrigerator, there's a decrease in the entropy of the kitchen/refrigerator combination. But the increase in entropy due to the additional heat produced in the refrigeration process will make up the difference (with room to spare for any realistic fridge). The second law of thermodynamics is safe!
Here's another example. At some point you've probably used a small hand pump to inflate a bicycle tire or a volleyball. When you do this, you're moving air molecules from a region where air is relatively sparse, to a container where the air is much denser. This represents a decrease in entropy. But just as with the refrigerator, this process can't take place on its own. It requires work on your part. Work that requires energy. And a byproduct of this process is, once again, heat. This heat represents an increase in entropy which more than makes up for the decrease in entropy due to the rearrangement of the air molecules, and the second law holds, as always.
So you see, the second law doesn't say that entropy can never decrease
anywhere. It says that the total entropy of the universe can never
decrease. Entropy can decrease somewhere, provided it increases somewhere
else by at least as much. That's the law.
Entropy and disorder
The Page of EntRoPy
 Back to Dave's Physics Shack?
Back to Dave's Physics Shack?


![]() Back to Dave's Physics Shack?
Back to Dave's Physics Shack?